3 Saint Elmo Brady
John Kaiser; Alex Meier; Kyler Luong; Soumi Vesali; and Shuai Sun
Saint Elmo Brady was the first African American to receive a Ph.D. in chemistry. Born on Dec. 22, 1884, in Louisville, Kentucky, Brady was the oldest of three children. After graduating from Louisville Colored High School in 1903, he left home at the age of 20 to attend Fisk University, an all-black college in Nashville, Tennessee. There he met and was encouraged to study chemistry by Thomas W. Talley. In 1908, Brady graduated from Fisk University with a bachelor’s degree, then took a teaching position in chemistry at Tuskegee University. He then took a leave of absence from Tuskegee Normal and Industrial Institute after teaching for four years when he was given a scholarship to University of Illinois at Urbana- Champaign. He started in the summer of 1913 and completed his M.S in chemistry in 1914. Brady’s Ph.D. research sought to examine how the acidity of straight chain carboxylic acids was affected when a pair of hydrogen atoms was replaced with an oxygen atom to give a keto acid.
Brady was the first to discover new methods for preparing and purifying certain compounds and was able to clarify the influence of carbonyl groups on the acidity of carboxylic acids. Brady was able to get his Ph.D. after two years, becoming the 40th person to receive a Ph.D. in chemistry from University of Illinois at Urbana- Champaign, and also the first African American chemist to earn a Ph.D. in the U.S. When first starting to get his Ph.D. there were 20 white males and he was the only black student. By the end of it, there were only 6 white males and himself getting a Ph.D. It was a struggle for Brady to find a home, for since he was African American he lived in segregated communities. During the time Brady was getting a PhD or even higher education it was very rare for someone other than a Caucasian getting or having the freedom to get higher education. During the time of the 6.3 % of African Americans that graduated high school only 1.2% of them got a bachelor’s degree and then of the 1.2% that got a bachelors only 1.8% got a master’s degree of the 1.2% that got a bachelor’s degree in the 1920s in the United States.
During this period of the 1920s, Brady was in the middle of a segregation area in the United States. During the segregation era especially since Brady was living in the South there was a heightened sense of discrimination. Before the segregation era, politicians developed the “separate but equal” phrase to “provide equal opportunities to African Americans”. “Separate but equal” would eventually be abolished because it was recognized to reinforce ideas of systemic oppression.
Brady’s research focused on various acids. He synthesized various carboxylic acids and analyzed the change in acidity when changing the group attached to the acid. Although this had not been termed yet, it is now known as the inductive effect, where the electronegativity of a connected atom can affect the acidity of an acid.
The acidity of a compound is measured by its pKa. This measures the ability of an acid to give away its proton to lead to what is called a conjugate base. The lower the pKa, the more acidic the compound. The more stable the conjugate base is, the more acidic the compound is. Once the proton is removed from an acid, it results in a negative charge on the conjugate base. A more electronegative atom connected to the acid helps compensate for this high negative charge by ‘pulling’ some of the electron density towards it. The distance between the electronegative atom and the acid also affects the pKa, and the closer the electronegative atom is to the acid, the large of an effect on the acidity it has. pKa is a logarithmic scale, which means that a change of 1 pKa unit will be a 10-fold change in acidity. pKa is also a measure of the pH at which 50% of the compound is protonated (acid) and 50% of the compound is deprotonated (base).
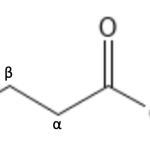
Brady’s work for his doctorate focused on synthesis of carboxyllic acids that had carbon chains, or an oxygen atom at the α, β, or γ position to the acid. The α, β, and γ are ways to measure the distance from a group on a compound. If a group is one atom away, it is considered the α position, two atoms away is the β position, and three atoms away is the γ position (Figure 1)
- Figure 1. α, β, and γ positions on a carboxylic acid
Brady’s work was the first to demonstrate what came to be known as the inductive effect on acidity. This is one of the four primary parameters that are used when attempting to identify the pKa of a compound. Despite performing his work in the 1800s, he was still able to discover an effect that is taught to undergraduate students every year.